Key Points:
- The researchers administered human neural stem cell vesicles (extracellular vesicles) via a nasal spray to explore its effects on mice modeling Alzheimer’s disease.
- The nasal spray preserved cognition and lowered the abundance of harmful brain protein plaques.
- The spray lowered brain immune cell—microglia—inflammatory response all the while preserving their capabilities to remove harmful protein accumulations—amyloid plaques.
Published in the Journal of Extracellular Vesicles, Shetty and colleagues from Texas A&M University show that a nasal spray containing human neuronal stem cell-derived extracellular vesicles helped preserve cognition in an Alzheimer’s disease mouse model. Moreover, the spray reduced harmful brain protein plaques. At the molecular level, the spray lowered the inflammatory response of microglia, all the while preserving their ability to remove damaging proteins. The study may pave the way for a new nasal spray treatment option for people with Alzheimer’s disease, the leading cause of death for those aged 65 and older.
Research showing the efficacy of this treatment against Alzheimer’s disease in mice has already precipitated further studies, according to a press release. Furthermore, Dr. Shetty has filed a patent for the nasal spray to treat Alzheimer’s and other neurological disorders. Dr. Shetty also hopes successful research on the spray could facilitate its application to delay severe cognitive decline associated with Alzheimer’s by 10 to 15 years.
“Our journey to advance the application of this therapy for Alzheimer’s disease is just beginning,” says Dr. Shetty.
A Nasal Spray that Preserves Cognition and Reduces the Harmful Buildup of Protein Plaques
Due to a lack of effective treatments that can delay the progression of Alzheimer’s disease, Shetty and colleagues explored the effects of their novel nasal spray therapy in mice modeling Alzheimer’s. They rationalized that since Alzheimer’s is tied to neuroinflammation and subsequent neurodegeneration, anti-inflammatory extracellular vesicles inhaled nasally may alleviate Alzheimer’s-related brain pathology.
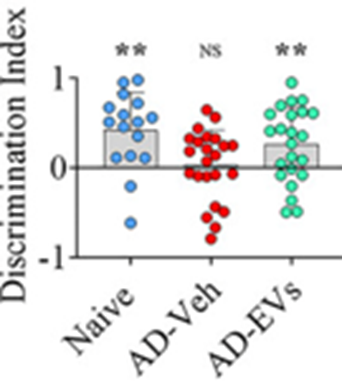
Indeed, after administering the extracellular vesicle-containing nasal spray to the Alzheimer’s mouse model, the mice showed preserved cognitive function. To measure cognition, the mice were placed in a square enclosure with objects they had been trained to recognize. When an object was placed in a new region of the enclosure, typical mice with normal cognition would spend more time examining the newly-located object.
The untreated Alzheimer’s-modeling mice with deteriorating cognition spent lower amounts of time inspecting the object that was moved to a new location, suggesting, as expected, cognitive decline. Mice modeling Alzheimer’s given the nasal spray, however, showed preserved cognition. These results provide preclinical evidence that the nasal spray therapy from Shetty and colleagues works against Alzheimer’s-related cognitive decline.
To uncover how the nasal spray preserves cognition in the Alzheimer’s mouse model, Shetty and colleagues turned to a key contributor of neurodegeneration—neuroinflammation—which comes in part from microglia. Interestingly, they found that the nasal spray reduced the prevalence of inflammatory protein complexes called inflammasomes emanating from microglia in the brain.
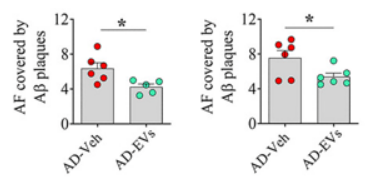
Also, since a buildup of protein plaques, known as amyloid plaques, serves as a hallmark of Alzheimer’s, Shetty and colleagues sought to find whether the nasal spray affects them. Interestingly, they found that the intranasal spray significantly reduced the abundance of amyloid plaques. Since microglia have been shown to engulf and remove amyloid plaques, it is possible that reducing neuroinflammation with the nasal spray enhances microglia propensity to remove the Alzheimer’s-related plaques.
The Nasal Spray May Prolong Effective Microglia Function
According to Dr. Shetty, the activation of microglia causes inflammation and the clearance of Alzheimer’s-related plaques from the brain. Thus, while the microglial response is initially helpful in removing damaging proteins, it can become problematic over time, precipitating neuroinflammation and subsequent neurodegeneration.
“Prolonged activation causes them to lose their normal function and begin to harm neurons, leading to progressive neuron loss,” says Dr. Shetty on the nature of over-activating microglia.
Therefore, the intake of human neural stem cell-derived extracellular vesicles via the nasal spray appears to lower the inflammatory response from microglia and allow them to continue removing Alzheimer’s-related plaques. Such a scenario may well impede inflammation-related neurodegeneration, which could help explain why mice modeling Alzheimer’s given the nasal spray showed preserved cognitive function.
As for when patients with Alzheimer’s disease could reap the benefits of such a nasal spray, it is still unclear when a human trial for the spray will get underway. Once human trials do start, though, if successful, it typically takes about 10 to 15 years for a new therapy to receive FDA approval. Therefore, while this study may propel a new, effective way to counter Alzheimer’s disease progression, it may take some time for this therapy to reach the market.