Key Points
- NAD+ transporter levels are elevated in the pancreas of type 2 diabetes patients.
- NAD+ levels decline with age in multiple compartments of pancreas cells.
- Decreasing NAD+ transporter levels reduces senescent cells, improves insulin signaling, and lowers blood glucose.
Some of our cells are more important than others, particularly when it comes to losing them during aging. Like losing a piece of ourselves, the loss of some cells brings us one step closer to chronic diseases such as diabetes. Diabetes, characterized by high blood glucose levels, occurs for two primary reasons:
- The pancreas’s insulin-secreting cells — β cells — become dysfunctional
- Cells become less sensitive to insulin and take up less glucose
In search of a novel therapeutic target for diabetes, researchers from Harvard Medical School and Seoul National University of Medicine in Korea conducted a study focusing on the progression of β cell dysfunction during aging. When it comes to stopping or treating diabetes, maintaining healthy β cells is paramount. However, research shows that with increasing age, type 2 diabetes, and higher body mass index (BMI), some β cells enter a dysfunctional state known as senescence.
Senescent Cells
Whether it be in the brain, skin, or pancreas, our cells are susceptible to biological stressors such as DNA damage. In response to these stressors, some cells cease to function normally, entering a state called senescence. With age, senescent cells accumulate and have been found to contribute to nearly all age-related chronic diseases by secreting inflammatory and tissue-degrading molecules. For this reason, scientists have been investigating compounds that remove senescent cells for the treatment of aging.
NAD+ Transporter Levels Elevated in Type 2 Diabetes
Along with senescent β cells, changes in NAD+ metabolism have been observed in the liver, heart, muscle, and β cells of individuals with prediabetes, diabetes, and obesity. Recent studies suggest that SLC25A51, a protein that transports NAD+ into mitochondria, accumulates in senescent β cells.
To elucidate the link between NAD+, senescence, and age-related type 2 diabetes, the Harvard scientists, led by Dr. Nora Kory, measured SLC25A51 protein levels from the β cells of mice. They found that SLC25A51 accumulates with age, specifically in β cells and not other pancreatic cell types. Moreover, SLC25A51 accumulation correlates with senescence, increased body weight, and higher blood glucose levels, suggesting it contributes to senescence and diabetes.

According to previous studies, developing interventions that prevent β cell senescence holds promise for the treatment of type 2 diabetes. Since SLC25A51 was found to be associated with β cell senescence in mice, the researchers next determined if high SLC25A51 levels are associated with type 2 diabetes in humans. They found that SLC25A51 mRNA levels were higher in male type 2 diabetes patients compared to healthy males, suggesting this mitochondrial NAD+ transporter accumulates in the β cells of individuals with type 2 diabetes.
NAD+ Levels Still Decline with Age
Further experiments showed that elevated SLC25A51 levels in senescent β cells are not a compensatory response or adaptation to lower NAD+ levels. The researchers confirmed that total NAD+ levels in the pancreas decline with age. Moreover, they showed that NAD+ levels decline in the cytosol as well as in mitochondria, suggesting that increases in SLC25A51, which transport NAD+ from the cytosol into mitochondria, do not compensate for age-related mitochondrial NAD+ decline.
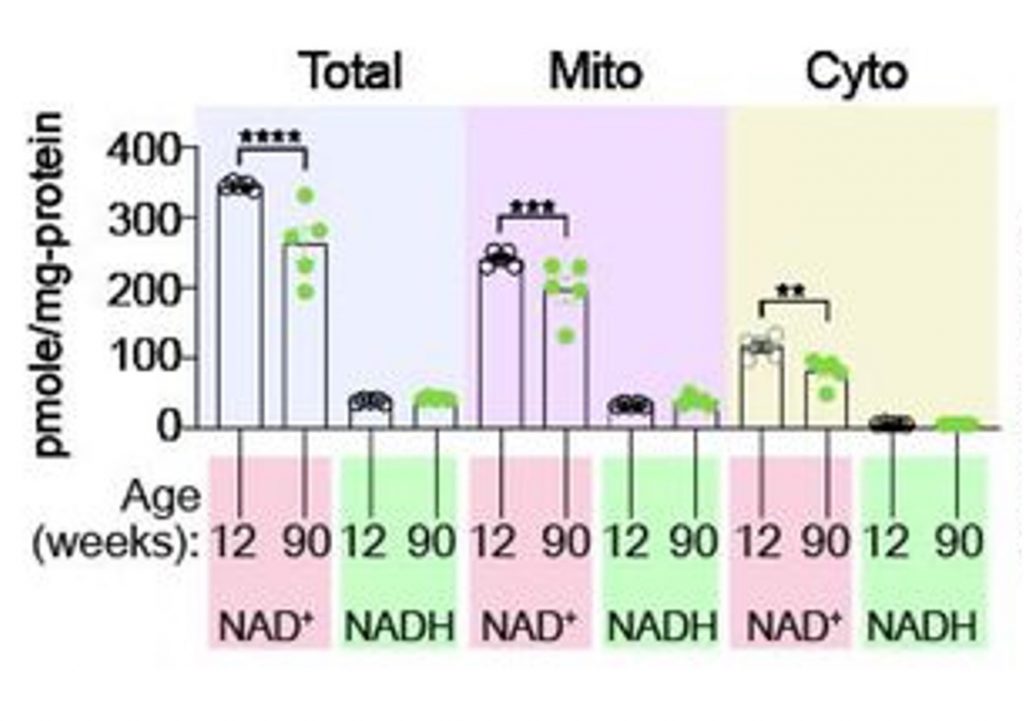
Instead, the researchers speculate that elevated SLC25A51 levels are just one of the necessary changes that senescent cells undergo to maintain their senescent state. For example, SLC25A51 is involved in glutamate metabolism, which senescent cells need to produce energy and survive.
Deleting NAD+ Transporter Mitigates Senescence and Diabetes
Like senescent cells, low NAD+ levels are associated with nearly every age-related chronic condition, including type 2 diabetes. In search of a therapeutic target, the Harvard researchers genetically deleted the SLC25A51 NAD+ transporter from the β cells of mice. The gene was deleted when mice were 30 weeks old, roughly equivalent to a 33-year-old human. They were then examined for 15 weeks until they reached the estimated age equivalent of a 40-year-old human.
The results showed that deleting SLC25A51 reduced senescent cell levels and slightly lowered blood glucose levels at certain time points. The SLC25A51-deleted mice also displayed more efficient glucose clearance from the blood than normal mice, suggesting improved insulin sensitivity. Fasting glucose and insulin levels further suggested improved insulin sensitivity. Considering that high blood glucose levels and poor insulin sensitivity are hallmarks of diabetes, these findings suggest that removing SLC25A51 could mitigate diabetes.

Senolytics for the Treatment of Diabetes and Beyond
Diabetes is a major risk factor for the premature development of age-related chronic conditions, such as cognitive impairment, cardiovascular disease, and kidney dysfunction. Diabetes itself can also promote the accumulation of senescent cells, as high blood glucose (hyperglycemia) is one of the biological stressors that induce senescence. Dr. Kory and the Harvard researchers have shown that countering senescence by removing SLC25A51 ameliorates symptoms of diabetes, supporting the idea that removing senescent cells can potentially contribute to treating diabetes.
With that being said, in their search for treatments against aging, scientists have identified compounds called senolytics, which selectively eliminate senescent cells. In animal models of diabetes, particularly obesity-induced diabetes, senolytics have been shown to improve insulin sensitivity and reduce cardiac dysfunction, liver inflammation, and obesity-induced anxiety. Of clinical relevance, in a study of individuals with diabetic kidney disease, the senolytic combo dasatinib and quercetin (D+Q) led to reduced circulating inflammatory markers, suggesting reduced inflammation.
Moreover, senolytics like D+Q and fisetin have been shown to prolong the lifespan of mice, suggesting they not only mitigate diabetes and other chronic diseases but also extend the lifespan of mammals. Whether senolytics can treat diabetes in humans will require more research. Since senescent cells spread senescence to surrounding cells, it may be better to lose a few senescent β cells to senolytics and save the rest from being lost to aging. Additionally, considering the age-related decline in pancreas NAD+ levels, taking an NAD+ precursor like NR (nicotiamide riboside), NMN (nicotinamide mononucleotide), or no-flush niacin could potentially mitigate diabetes as well.