Key Points:
- Senescent immune cells increase in the brain with age.
- Eliminating senescent cells reduces pro-inflammatory immune cells in the brain.
- Treatment also helps prevent the loss of executive functioning skills – skills that help us plan, focus, remember, and juggle multiple tasks.
Increased inflammation is a critical symptom of aging, linked to cognitive decline and various age-related concerns. A new study shows that clearing senescent immune cells – aged cells which are permanently in a non-proliferating state and induce inflammation – from the brain may hold the key to preventing age-related cognitive issues.
The study, out of the Mayo Clinic, published in Nature Communications, focused on senescent immune cells in the brains of mice. The researchers found that senescence and inflammation increase in the brain with age. Immune cells that reside in the brain (microglia) and those that have migrated into the brain (monocytes) showed increased senescence and pro-inflammatory tendencies. When these senescent cells were removed, the mice had increased nesting capabilities, indicative of increased executive functioning.
“Our study reveals dynamic remodeling of the brain immune cell landscape in aging and suggests senescent cell targeting as a strategy to counter inflammatory changes and cognitive decline,” the investigators wrote.
Rejuvenating the Brain Landscape for Preventing Cognitive Decline
The scientists looked at 6-month-old (approximately 34 human years) and 24-month-old mice (approximately 70 human years) mice. The investigators found that genes involved in senescence and inflammatory processes in the brain increased with age. They also found decreased neurotransmitter genes, which in conjunction with increased inflammation and senescence, may lead to decreased cognition.
Senescence is thought to exist to repair wounds and prevent cancer, however, senescent cells accumulate with age and promote inflammation. Senescent cells also signal other immune cells like monocytes (immune cells in the bloodstream) to their location, increasing the inflammatory response.
Both pro-inflammatory monocytes and microglial cells (brain immune cells) were found to have increased senescent genes in the old mice, suggesting that both resident (microglia) and infiltrating (monocytes) cells contribute to the brain’s pro-inflammatory, senescent environment. Further analysis showed that pro-inflammatory immune cell abundance is strongly related to cognitive dysfunction.
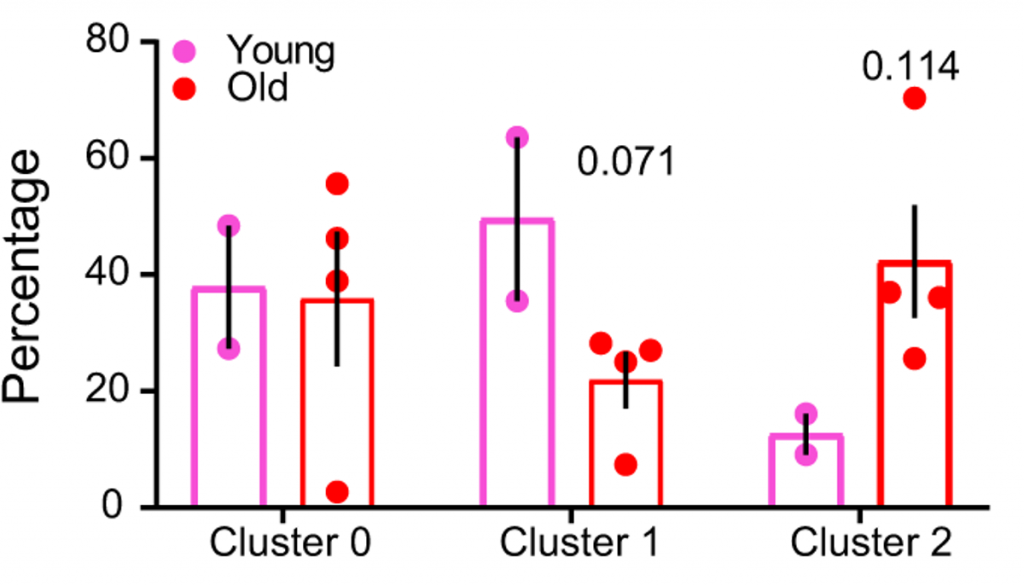
The scientists chose to focus on female mice because of their more pronounced senescent cell gene signature. The mice used in the study were genetically modified to have senescent cells activate a protein sensitive to a drug called AP20187. In response to AP20187, the modified protein activates cell death, allowing for the selective elimination of senescent cells.
Upon senescent cell elimination, mice exhibited reduced brain inflammation. The senescent cell clearance also helped restore infiltrating immune cells to more youthful levels. Furthermore, cognitive function was restored, with the treated mice able to build nests – a measure of executive function – with the same quality and speed as younger mice. Maze studies also showed improvement in spatial learning and memory based on decreased mistakes.
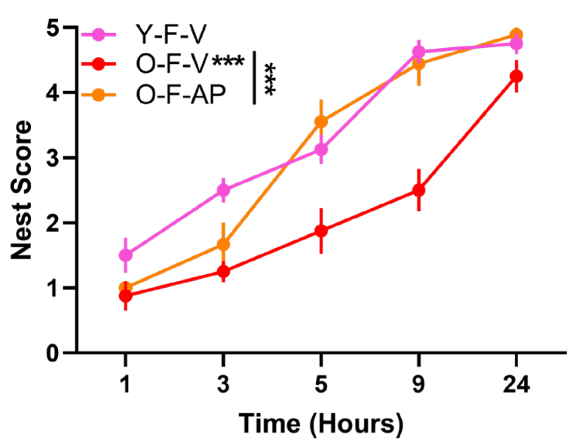
Senolytics for Cognitive Decline
Zhang and colleagues show how clearing senescent cells may help prevent cognitive decline by decreasing inflammatory cells in the brain. Previous studies have shown that senolytics can increase memory in mice, and other studies have shown how inflammation decreases with senolytic treatment following organ transplants. Senolytics are small molecules that selectively eliminate senescent cells. Thus, these studies also support the idea that removing senescent cells in the brain alleviates inflammation to restore cognitive function.
Many senolytic treatments are used as cancer treatments, but with more research, we may see senolytics being used as anti-aging treatments to prevent cognitive decline and relieve the inflammation that tends to increase as we age.