Key Points:
- “Trojan Horse” nanoparticles selectively target inflammatory macrophages – a type of white blood cell that protects the immune system by removing pathological cells.
- Treating pigs modeling atherosclerosis with these nanoparticles reduces inflammation and the accumulation of dying cells in arteries.
- The nanoparticle treatment does not induce anemia like previous antibody treatments targeting atherosclerosis.
Nearly 18 million people worldwide die every year from atherosclerosis, a progressive condition characterized by arterial wall plaque buildup. Notably, scientists have pinpointed chronic inflammation as a major factor exacerbating plaque formation, making it a primary target for novel therapeutics. Currently, researchers have attempted to thwart arterial inflammation with treatments activating a biological process called efferocytosis, which involves removing dysfunctional or apoptotic (dying) cells by a type of white blood cell (macrophages).
Studies have shown that impaired efferocytosis in atherosclerotic plaque leads to larger necrotic cores – a mass of dead vascular cells – that increase susceptibility to plaque rupture and blood clots. This happens, in part, due to the increased activity of a surface protein called CD47, which acts as a “don’t eat me” signal. When activated, it effectively hinders the clearance of apoptotic vascular cells
In preclinical studies, blocking CD47 with monoclonal antibodies reactivated efferocytosis, successfully reducing plaque burden. However, in a phase 1 clinical trial, results showed that blocking CD47 had unintended consequences. Instead of just removing apoptotic vascular cells, the treatment also unintentionally removed aged red blood cells from the spleen, causing anemia in subjects.
To overcome this, researchers developed a novel nanotherapy, dubbed “Trojan Horse” nanoparticles, that utilizes a chemical inhibitor to bypass CD47 toxicity and reactivate macrophage efferocytosis selectively within atherosclerotic plaques. Remarkably, rodent studies revealed that the nanotherapy precisely targeted inflammatory macrophages within the plaque, boosting efferocytosis and reducing plaque burden. Importantly, the treatment did not induce anemia like previous antibody treatments.
Now, researchers from Stanford School of Medicine published a study in Nature Communications investigating whether the promising results observed in rodent models could be replicated in a larger animal model (pigs), aiming to assess the treatment’s feasibility for future clinical trials.
Nanotherapy Selectively Targets Inflammatory Macrophages
The study utilized genetically modified pigs modeling atherosclerosis to assess the targeted delivery and protective effects of the novel nanotherapy. To assess the nanotherapy’s capacity for targeted delivery, the investigators treated pigs with two forms of the nanotheray: one carrying the chemical inhibitor and one without the chemical inhibitor (control group).
After 12 weekly infusions of the nanotherapy, the researchers examined the inflammatory macrophages to determine which treatment led to the highest concentration of the nanotherapy. They found that the nanoparticles carrying the chemical inhibitor accumulated in inflammatory macrophages at significantly higher concentrations compared to the pigs treated with the nanoparticles without the chemical inhibitor. Accordingly, the results demonstrate that the chemical inhibitor enhances targeted delivery to inflammatory macrophages.
Nanotherapy Reduces Vascular Inflammation and Apoptotic Cell Accumulation
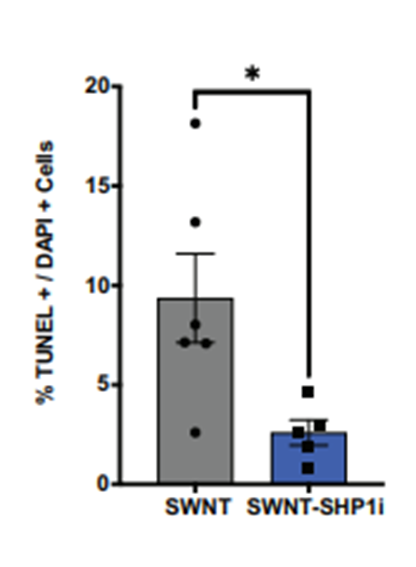
Following the nanotherapy’s assessment for targeted delivery, the research team evaluated the treatment’s impact on vascular inflammation and apoptotic cell accumulation. Images of the carotid arteries revealed that pigs treated with the nanoparticles carrying the chemical inhibitor exhibited significantly less vascular inflammation and fewer apoptotic cells. Collectively, the results suggest that the novel nanotherapy effectively thwarts atherosclerotic inflammation and enhances efferocytosis, as evidenced by the decreased number of apoptotic cells.
Can We Expect Clinical Trials Soon?
Bemezai and colleagues sought to identify a treatment that would enhance targeted efferocytosis in atherosclerotic plaque without inducing the clearance of healthy cells, a limitation observed with current antibody treatments. Fortunately, the study revealed that the “Trojan Horse” nanoparticles did not induce off-target efferocytosis or trigger anemia, highlighting the treatment’s safety.
Overall, the study’s findings provide strong support for the feasibility of future clinical trials with the “Trojan Horse” nanoparticles, which may one day serve as a viable treatment for cardiovascular disease. That being said, clinical trials take roughly 10 to 15 years on average to reach completion. This means we should focus our attention on steps we can take today to mitigate the risk of atherosclerosis, such as performing regular exercise, stopping smoking, and eating heart-healthy foods like vegetables, fruits, whole grains, and lean proteins.