Key Points:
- Researchers have made substantial progress in delaying the aging of animal models by targeting the hallmarks of aging, such as the accumulation of dysfunctional cells (known as senescent cells) and inflammation.
- Finding whether agents targeting the hallmarks of aging, such as aspirin, metformin, and NAD+ precursors, also delay aging in humans is just beginning to gain speed.
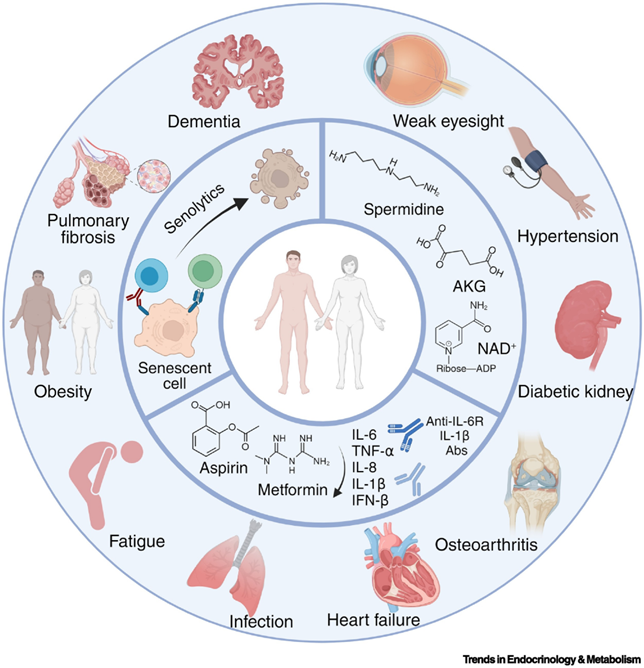
The identification of the hallmarks of aging—characteristics of age-associated physiological decline that therapeutics can target to slow or reverse aging—has served as a crucial step in developing aging intervention technologies. Reported in Trends in Endocrinology and Metabolism, Liu and colleagues from Shenzhen University Medical School in China relay data showing that some interventions targeting the hallmarks of aging extend the lifespan of animals, as well as new human trial data testing their efficacy.
Such aging interventions include senolytics (compounds that remove senescent cells), drugs that can ease chronic inflammation, and metabolism-mediating molecules within cells that may have aging intervention properties. The specific technologies reviewed for each type of aging intervention are dasatinib and quercetin for senolytics; aspirin and metformin for agents that counter inflammation; and nicotinamide adenine dinucleotide (NAD+) precursors for metabolites with aging intervention properties.
Exploring Aging Intervention with Senolytics
For starters, senolytics selectively eliminate senescent cells—cells that accrue in tissues with age that are characterized by their arrested proliferation, resistance to programmed cell death (apoptosis), and their secretion of proinflammatory molecules. The accumulation of senescent cells has been associated with various age-related diseases. For this reason, selectively eliminating this type of cell with senolytics has been proposed as a means to possibly delay aging.
Remarkably, in a study where researchers treated aged mice with the senolytics dasatinib and quercetin, this senolytic combination significantly extended lifespan. Additionally, this combination alleviated physical dysfunction in the aged mice.
These findings have paved the way for human studies testing the possibility that senolytics prolong years without disease (a concept known as healthspan) for people. Along those lines, a human trial assessing the safety and feasibility of using senolytics has been conducted on patients with a disease that causes lung scarring called idiopathic pulmonary fibrosis (IPF). In these patients, following three weeks of intermittent dasatinib and quercetin treatment, notable improvements in physical function were seen. These observed improvements highlight the potential of senolytics to improve physical function in patients with IPF, a condition associated with premature lung aging.
Another human trial testing dasatinib and quercetin in an age-related disease examined their effects in patients with diabetic kidney disease. Researchers found that administering dasatinib and quercetin for three consecutive days reduced senescent cell abundance in skin and fat tissues in that study. Moreover, the dasatinib and quercetin treatments in that study were associated with a reduction in proinflammatory proteins, such as IL-6, in blood plasma.
Finally, a study examining bone metabolism in postmenopausal women was conducted, which showed an association between dasatinib and quercetin treatment and an increase in blood plasma P1NP—a protein marker for bone formation.
Despite these mouse studies and human trials illustrating a high degree of potential for senolytics like dasatinib and quercetin, further human trials are necessary to investigate in which tissue types and organs senolytics effectively eliminate senescent cells. Accordingly, some senolytics may work more effectively in some organs than others. Moreover, more comprehensive human trials testing senolytics’ capabilities to ameliorate age-related diseases are necessary to confirm their efficacy against aging.
Targeting Age-Related Chronic Inflammation
Chronic inflammation is recognized as one of the hallmarks of aging. Along those lines, targeting inflammation may serve as a way to prolong healthspan and possibly even lifespan. Promisingly, using anti-inflammatory interventions in animal models has been shown to alleviate aging-related conditions and extend lifespan.
For example, research has shown that using drugs to reduce levels of a small protein that regulates inflammation called TNF-ɑ prevents age-related muscle degeneration in aged mice and alleviates age-related cognitive impairment in rats. Moreover, in prematurely aged rats (progeroid rats), reducing a gene for a protein complex that initiates inflammation—NLRP3—extended their lifespans. Such preclinical research suggests that lowering inflammation may serve to extend healthspan and maybe even lifespan in humans.
As for human studies of anti-inflammatory interventions, long-term use of the anti-inflammatory agent aspirin was associated with a 33% reduction in the incidence of colorectal cancer and a lowered mortality rate. Another large-scale analysis showed an association between the regular use of anti-inflammatories similar to aspirin (called NSAIDs) and a 20% lower risk of Alzheimer’s disease. How NSAIDs like aspirin alleviate inflammation needs clarification, and the potential for the regular use of aspirin to extend healthspan in humans needs further clinical verification.
Another drug, used to treat diabetes, that lowers inflammation is metformin, which may be used to counter a range of age-related diseases. Evidence supporting the possibility that metformin treats multiple conditions arising from aging comes from research showing its use was associated with a reduced all-cause mortality in patients with diabetes, even conferring a lower all-cause mortality compared to individuals without diabetes. Interestingly, metformin suppresses the production of TNF-ɑ and inhibits NLRP3 and also influences other proinflammatory pathways to reduce inflammation. The research on metformin positions the anti-diabetes drug as a promising aging intervention with possible effects against aging beyond diabetes.
Thus, targeting inflammation with drugs like aspirin and metformin may serve as a way to slow aging. Once more human trial data comes to light, we will have a better handle on whether antiinflammatories extend healthspan and lifespan and what doses are most optimal.
Metabolism-Modulating Molecules and Their Precursors to Optimize Metabolism
Metabolism—encompassing all the chemical reactions in cells that convert food to energy—is a fundamental biological aspect of all organisms. Chemical byproducts of metabolism are called metabolites, and many have been implicated in age-related diseases. Furthermore, some, such as NAD+, have been identified as mediators of aging. Through supplementing metabolism-modulating molecules like NAD+, some researchers have aimed to combat aging.
Research suggests that cellular NAD+ falls with age across animal models of aging and humans. Not only that but preclinical studies have demonstrated that supplementing with NAD+ precursors like nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR) positively influences healthspan in mice. These preclinical findings hint that humans may increase cellular NAD+ levels with precursors to possibly prolong healthspan.
As for human trials with NAD+ precursors, NMN and NR have been tested and shown to be safe and tolerable when taken orally in several studies. Another trial associated NMN supplementation with improved muscle insulin sensitivity in prediabetic, postmenopausal women. Additionally, a human study associated NMN supplementation with enhanced submaximal exercise performance in amateur runners. These studies, together with others showing that NAD+ precursors increase blood NAD+ in humans, suggest that supplementing with NMN or NR may improve physiological indicators related to healthspan.
Despite these findings, though, data precisely linking increased blood NAD+ from using precursors to specific enhancement of physiological functions remains scant. For this reason, more human trials that include larger numbers of subjects will be essential for addressing this gap in knowledge.
Finding Ways to Address Multiple Hallmarks of Aging Simultaneously
Researchers have made substantial advancements in understanding what underpins aging over the past few decades. Perhaps most importantly, the identification of certain hallmarks of aging has provided scientists with biological targets to slow aging’s progression.
In that regard, certain hallmarks of aging have been targeted with agents like NAD+ precursors and other therapies to efficiently delay aging and related disorders in animal models. Although human trials testing whether these aging interventions can translate to humans are advancing rapidly, they are still in their beginning phases. As such, most human trials examining these interventions remain in phases testing the safety, tolerability, and feasibility of the treatments.
Perhaps completion of human trials linking some of these aging interventions to the improvement of physiological parameters related to healthspan will be completed in the next 5 to 10 years. While we are all waiting for data confirming the efficacy of such treatments, some nutraceuticals contain multiple technologies that target more than one hallmark of aging.
For example, some contain senolytics for the selective elimination of senescent cells, the buildup of which constitute one of the hallmarks of aging. Others have components that stimulate autophagy—the breakdown of cellular waste and recycling of certain cellular components—the dysfunction of which is another hallmark of aging. Additionally, NAD+ precursors in some nutraceuticals can increase NAD+ as we age.
By targeting multiple hallmarks of aging, nutraceuticals containing these components, such as RESTORIN, may serve to stave off the ravages associated with getting older. As further data emerges, we may better understand the potential of these technologies to enhance healthspan and possibly extend lifespan. In the meantime, preclinical trials have shown that some of these components like senolytics enhance physiological parameters of healthspan and extend lifespan in animal models.