Key Points:
- Canagliflozin reduces the prevalence of dysfunctional cells that accumulate with age (known as senescent cells) in mice fed a high-fat diet.
- This diabetes medication also reduces fat tissue inflammation.
- Canagliflozin extends the lifespan of mice with a condition that induces premature aging called progeria by 12%.
Published in Nature Aging, Minamino and colleagues from Juntendo University in Japan have shown for the first time that a diabetes medication called canagliflozin clears senescent cells in mice fed a high-fat diet. The diabetes drug also alleviates fat tissue inflammation.
Moreover, Minamino and colleagues found that canagliflozin extended lifespan in a mouse model for premature aging — progeria. There is evidence that the accumulation of senescent cells plays a significant role in premature aging for patients with progeria. In this regard, it is plausible that canagliflozin alleviates an overabundance of senescent cells in mice with progeria to extend their lifespans.
Canagliflozin is a medication prescribed to diabetes patients and is meant to help control high blood sugar. This FDA-approved drug increases the amount of sugar excreted in urine, thereby lowering blood glucose levels. According to the findings from Minamino and colleagues, though, canagliflozin may also stave off some of the effects of aging that stem from senescent cell-associated inflammation.
Adding to the alluring nature of these findings is how canagliflozin clears senescent cells — by activating a protein complex that regulates energy homeostasis called AMPK. Interestingly, other AMPK activators, such as the nicotinamide adenine dinucleotide (NAD+) precursor nicotinamide mononucleotide (NMN) and the diabetes medication metformin, have also been shown to confer senescent cell clearing activity. The findings from Minamino and colleagues beg the question of whether AMPK activators in general could be used for senescent cell clearance.
Canagliflozin Clears Senescent Cells and Extends Lifespan
Restricting calorie intake (known as calorie restriction) has been shown to extend the lifespan of various organisms and is associated with a decreased accumulation of senescent cells. Because canagliflozin leads to calorie loss through the excretion of glucose in urine, the Japan-based researchers speculated that canagliflozin could also clear senescent cells, in a way similar to calorie restriction. For this reason, the researchers measured canagliflozin’s effects on senescent cells in fat tissue.
Interestingly, while a high-fat diet induced higher levels of senescent cells in fat tissue, when the researchers administered canagliflozin, their levels significantly declined. As these results suggest, canagliflozin may have the added benefit of senescent cell clearance, similar to the effects of calorie restriction, along with controlling blood glucose levels.
Additionally, the researchers’ results suggest that canagliflozin works differently from the common diabetes medicine insulin. As such, the Japan-based researchers found that insulin did, in fact, lower blood glucose; however, it had no effect in the way of clearing senescent cells. This finding also indicates that canagliflozin’s senescent cell-clearing effects do not come from lowering blood glucose.
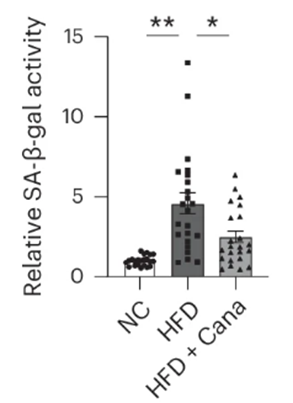
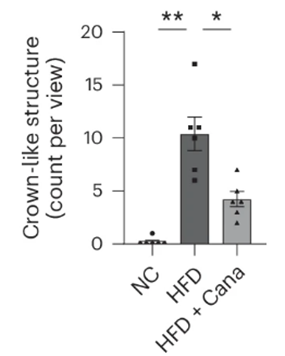
Since some senescent cells emit inflammation-promoting molecules called the senescence-associated secretory phenotype (SASP), Minamino and colleagues tested whether canagliflozin also quells inflammation. They turned to fat tissue for this assessment and found canagliflozin indeed reduced inflammation. Thus, possibly through inducing the clearance of senescent cells, canagliflozin does, in fact, reduce fat tissue inflammation.
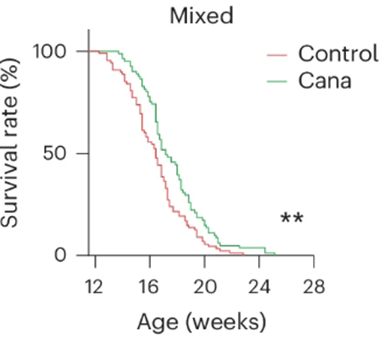
The buildup of senescent cells has been associated with premature aging in progeria patients. For this reason, Minamoto and colleagues sought to find whether canagliflozin’s senescent cell clearing capabilities had an effect on lifespan in a mouse model for premature aging — mice with progeria. Interestingly, canagliflozin extended the median lifespan modestly by about 12%. Thus, it seems plausible that canagliflozin extended progeria mouse lifespan through senescent cell clearance.
AMPK Activators May Clear Senescent Cells
Minamino and colleagues also found that canagliflozin could not clear senescent cells when they blocked the activation of the energy-regulating protein complex AMPK. This suggests that canagliflozin clears senescent cells through the modulation of AMPK.
In addition to this finding, other research suggests that other AMPK-activating compounds, such as NAD+ precursors and metformin, clear senescent cells. Thus, it may be the case that compounds that activate AMPK work to stimulate the immune system to remove senescent cells.
Research has also associated calorie restriction with AMPK activation and lifespan extension in some organisms. Thus, through their activation of AMPK, canagliflozin and other AMPK activators may partially mimic calorie restriction to eliminate senescent cells and extend lifespan in mice with progeria.
This also leads to the question of whether, generally speaking, AMPK activators mimic calorie restriction to alleviate age-related diseases and possibly extend lifespan through the clearance of senescent cells. A multitude of clinical trials are underway testing AMPK-activating NAD+ precursors’ capabilities to stave off cognitive, cardiovascular, and physical deterioration. In that sense, the verdict is still out as to whether AMPK-activating compounds like NAD+ precursors extend the number of years we live without disease — our healthspan.
What’s more, addressing whether AMPK activators also extend our lifespans will require time-consuming and potentially cost-prohibitive human trials. Along those lines, the Targeting Aging with Metformin (TAME) trial testing the AMPK activator and diabetes drug metformin on human lifespan still needs more funding for its execution.