Key Points:
- Egg cells (oocytes) in female mice fed a high-fat diet exhibit significantly lower levels of NAMPT, an enzyme that contributes to NAD+ biosynthesis.
- NAMPT insufficiency in obese mice triggers harmful defects, like increased oxidative stress, on oocytes, leading to problems with cell division.
- Supplementation with the NAD+ precursor nicotinic acid (NA) mitigates the effects of NAMPT depletion.
Having a baby is complicated, and hopeful parents must take the necessary precautions to avoid complications. To conceive a healthy baby — with five fingers and five toes — countless events need to be executed flawlessly, from the joining of sperm and egg for fertilization to the proper development and birth of the baby. However, none of this can occur if the quality of the egg, or what scientists call oocyte, isn’t where it should be. Of the many factors that influence oocyte quality, none are more in control of the bearer than our diets.
Wang and colleagues show in Aging Cell that obese mice fed a high-fat diet exhibit compromised oocyte quality, which is linked to significantly reduced levels of an enzyme critical for the biosynthesis of nicotinamide adenine dinucleotide (NAD+) — a vital energy-producing compound. What’s more, the investigators found that depletion of this enzyme, called NAMPT, led to cell division complications in oocytes and overproduction of ROS. But Wang and colleagues were able to flip this around by supplementing obese mice with an NAD+ precursor, nicotinic acid (NA), which boosted NAD+ levels, partially improved oocyte meotic obstructions, and mitigated oxidative stress. This study highlights the potential therapeutic use of NA or other NAD+ precursors to improve oocyte and embryo developmental capability.
Regulating Oocyte Health
Maintaining oocyte quality is paramount to a healthy pregnancy. Once an oocyte becomes compromised, the likelihood of an oocyte reaching full maturity (a fertilizable egg cell) drops dramatically. Before an oocyte achieves maturity, it needs to undergo meiosis — a cell division that minimizes the number of chromosomes in the parent cell by half to receive the other half from a male germ cell (sperm).
However, meiotic complications arise when certain parameters aren’t met. Upon cell division, protein structures known as spindles symmetrically align and pull the cell’s chromosomes into separate cells. However, improper chromosome alignment and malformed spindles can lead to asymmetric cell division and unbalanced chromosome numbers, compromising oocyte maturation.
While we already know that managing our diets is important for our own health, it may have major consequences on our offspring (or lack thereof). We can influence the success of the oocyte maturation process by what we consume. Our dietary choices significantly impact the execution and outcome of these processes necessary for fertilization.
For example, raw meats and foods high in mercury have been linked to compromised fetal development and neurological illnesses. In rodent models, researchers have shown that mice fed a high-fat diet exhibit altered spindle formation and damage to cellular power generators (mitochondria). More importantly, the presence of compounds called reactive oxygen species reaches toxic levels, leading to damaged DNA and potentially harmful mutations.
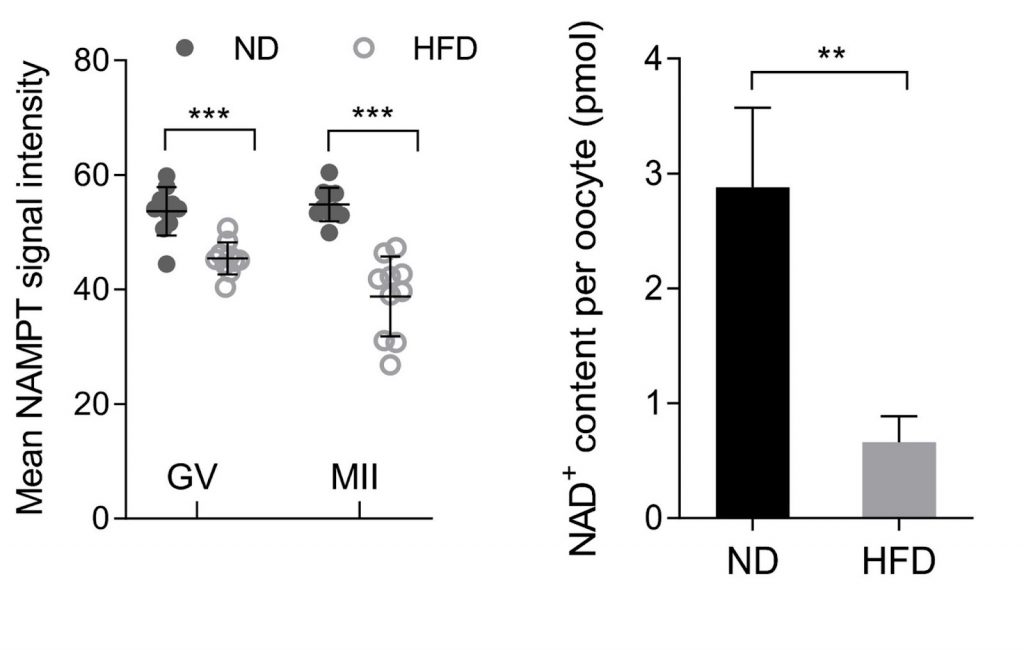
Oocyte NAMPT Expression is Lower in Mice Fed a High-Fat Diet
As previous studies have linked obesity to changes in NAMPT activity, Wang and colleagues compared NAMPT protein levels in oocytes from mice on a normal diet or high-fat diet. When comparing levels of NAMPT in oocytes, those from high-fat diet mice showed decreased levels of the NAD+ synthesis-related enzyme, suggesting that diets linked to obesity directly impact NAMPT activity in oocytes. Notably, diminished NAMPT leads to decreased NAD+ levels, which promotes various metabolic disorders, including obesity and type 2 diabetes.
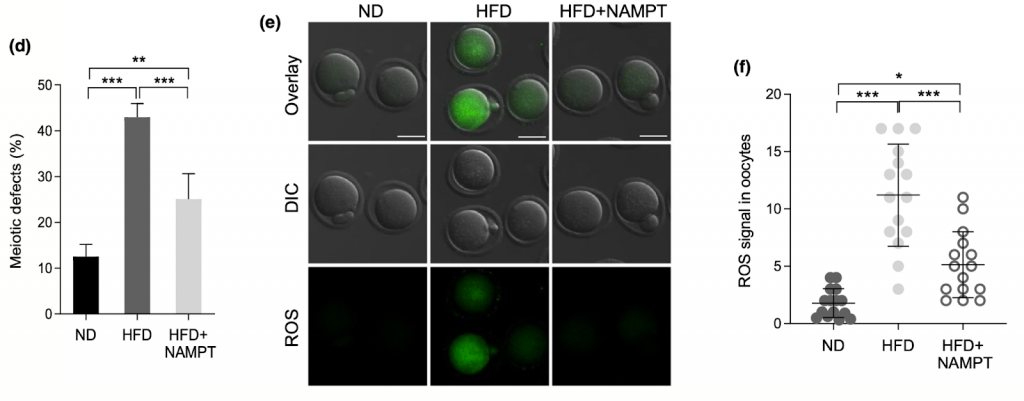
Replenishing NAMPT in HFD Oocytes Rescues Defects
Next, the Nanjing Agricultural University researchers wanted to look at the consequences of NAMPT depletion during oocyte maturation. Specifically, they looked at how, if at all, NAMPT impacted meiosis in oocytes. Upon examining spindle formation and chromosome parameters in high-fat diet oocytes during meiosis, Wang and colleagues detected compromised spindle formation and asymmetric division, which suggests impaired meiotic progression. What’s more, untreated control oocytes had significantly more NAD+ than high-fat diet oocytes, which likely has metabolic and energetic consequences. Also, consistent with previous studies, high-fat diet oocytes displayed higher levels of ROS, indicating increased oxidative stress.
Wang and colleagues wanted to know if, while still on a high-fat diet or with weak NAMPT activity, NAD+ replenishing compounds could help produce healthy, mature oocytes. First, the investigators manually increased NAMPT protein levels in high-fat diet oocytes. What they found was, compared to untreated mice on high-fat diets, improved spindle and chromosome alignment, elevated NAD+ levels, and less oxidative stress. The findings suggest that NAMPT deficiency hinders oocyte maturation, disrupts metabolism by reducing NAD+ levels, and elevates oxidative stress, all of which contribute to infertility and pregnancy complications.
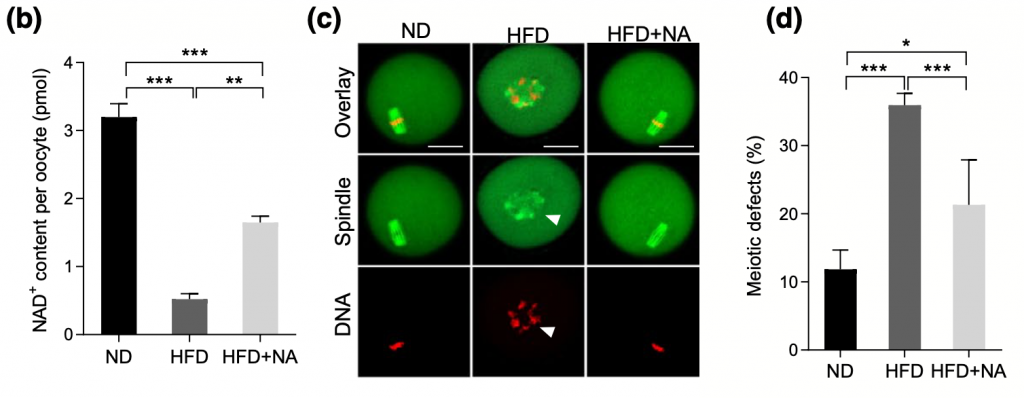
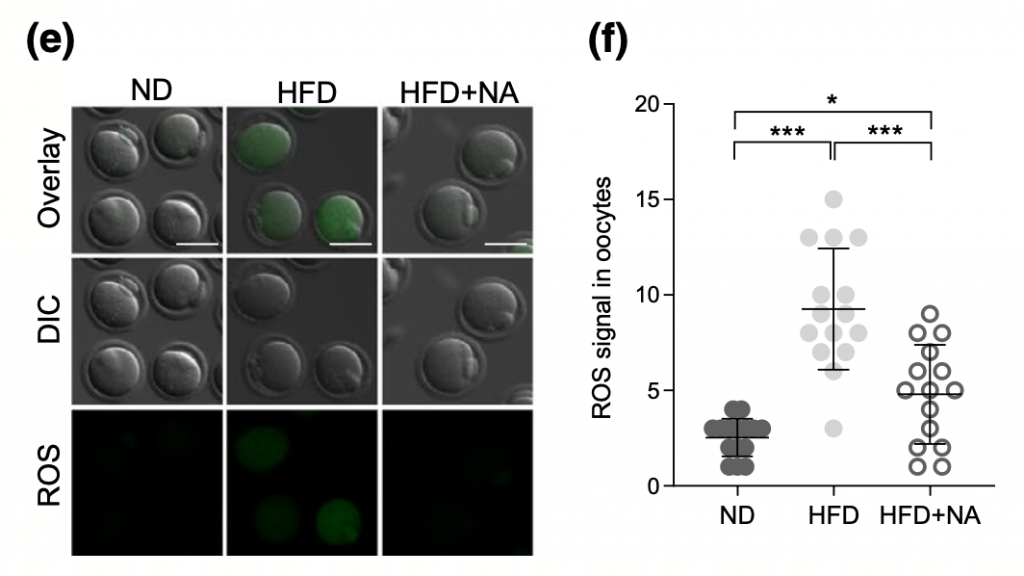
An NAD+ Precursor Mitigates Consequences of NAMPT Depletion in HFD Oocytes
Although higher levels of NAMPT ameliorated the characteristics observed in high-fat diet oocytes, Wang and colleagues wanted to evaluate the potential benefits of supplementing with an NAD+ precursor. After supplementing with the NAD+ precursor nicotinic acid (NA), oocytes from mice fed high-fat diets presented normal spindle and chromosome organization, higher NAD+, and diminished levels of ROS. It’s clear that the presence of NAMPT is critical for oocyte development, and these findings shed light on the possibility of utilizing an NAD+ booster to attenuate the potential complications of maternal obesity.
“In this study, by employing a high-fat diet (HFD)-based mouse model, we discovered a significant reduction of NAMPT protein and NAD+ content in oocytes from obese mice,” said Wang and colleagues. “Remarkably, our results show that both in vivo administration and in vitro supplement of nicotinic acid (NA) effectively ameliorate the obesity-associated meiotic defects and metabolic dysfunction in oocytes.”
What’s next for NAD+ precursors?
The detailed pathways controlling NAD+ production in mammalian oocytes remain unclear. Nevertheless, clarification of this issue will be important for identifying a better booster of oocyte quality. Ultimately, future systematic evaluation is required to determine whether/how NAD+ precursors influence metabolic phenotypes and reproductive outcomes of obese mice.