Key Points:
- Obesity due to a high-fat diet causes subfertility – fewer mature oocytes (eggs) and smaller litters in mice.
- Supplementing with nicotinamide riboside (NR), an NAD+ precursor, alleviates the obesity-induced subfertility.
- These findings suggest that NAD+ boosting through precursor supplementation could be a potential therapy for diet-induced subfertility.
Infertility is on the rise, currently affecting about 1 in 5 Americans, and nearly 1 in 3 Americans report that they or someone they know has gone through in vitro fertilization (IVF) to treat their infertility. Extremes on either end of the weight spectrum can lead to infertility and affect IVF success, which costs around $15,000 per cycle and has roughly a 50% failure rate. So, with obesity on the rise in modernized countries, particularly the U.S., and more people turning towards artificial reproductive technologies to get pregnant, there’s a lot to gain from improving fertility.
Recently, a study published in Clinical Translational Medicine from researchers in China found that increasing levels of NAD+ – a cofactor involved in multiple essential cellular processes – may improve subfertility (reduced fertility) in obese individuals. Mice fed high-fat diets have fewer oocytes (eggs) and offspring along with decreased NAD+, but supplementation with nicotinamide riboside (NR) (400 mg/kg/day), an NAD+ precursor, reverted some of the effects of obesity on fertility. NR supplementation supported fertilization by maintaining oocyte health by improving mitochondrial health as well as preventing cellular stress and detrimental changes to oocyte structure and genome organization caused by a high-fat diet. The improvements to mitochondria were seen in the resulting offspring of high-fat diet mice, as the new generation demonstrated improved metabolism.
“Our data suggest that increasing NAD+ level is a potential therapeutic approach for treatment of obesity-related ovarian infertility and metabolic disturbance in offspring,” wrote the authors.
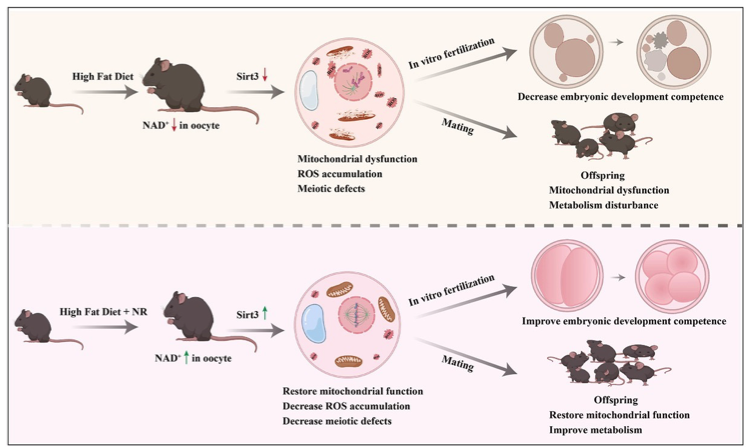
(Yang et al. (2021) | Clin Transl Med.) Nicotinamide Riboside (NR) supplementation alleviates obesity-induced subfertility and restores mitochondrial function in mothers and their offspring. Obesity decreases NAD+ levels in oocytes, which leads to mitochondrial dysfunction accompanied by reactive oxygen species (ROS) accumulation and defects in meiosis – cell division of cells the produce oocytes. These meiotic defects result in reduced oocyte quality and earlier embryonic developmental competence. NR supplementation restores mitochondrial functions in oocytes by restoring the NAD+ level and thus decreases ROS accumulation and meiotic defects via a Sirt3‐dependent pathway. Importantly, administration of NR attenuates the obesity‐induced decreases in early embryonic competence and the live birth rate as well as normalises the metabolism in offspring.
NR Boosts NAD+ Levels to Mitigate Subfertility Induced by a High-fat Diet
There are so many stages that need to go right for successful fertilization and pregnancy. If the DNA of the gametes – sperm and egg – isn’t healthy or properly organized into chromosomes, fertilization and the subsequent multitudes of rounds of cell division cannot occur successfully. These steps are dependent on NAD+, which helps protect the cell’s DNA and decrease cellular stress.
This study used four-week-old mice (equivalent to a 14-year-old human) fed a high-fat diet for three months. The high-fat diet fed mice became subfertile, having smaller litters (fewer offspring) than those fed normal chow. When the high-fat diet was supplemented with NR, fertility improved. The supplemental NR resulted in more oocytes and increased NAD+ levels in both the oocytes and ovaries of the mice fed a high-fat diet.
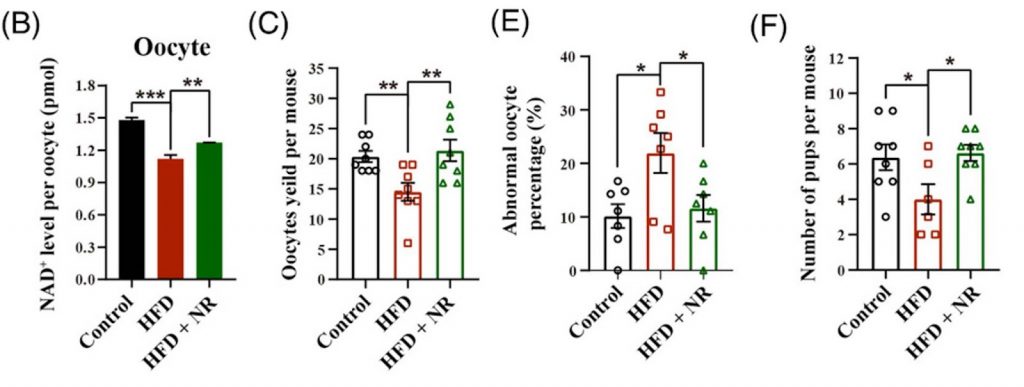
(Yang et al. (2021) | Clin Transl Med.) Supplementation of NR attenuates the obesity-associated decline in oocyte quality and improves early embryonic competence by increasing the NAD+ level. (B) NAD+ levels in oocytes were decreased in mice fed a high-fat diet, and NR supplementation mitigated these effects. (C) The number of oocytes per mouse decreased in those fed a high-fat diet, and NR supplementation took the levels back to control levels. (E) More abnormal oocytes were observed in mice fed a high-fat diet than in control, but these levels were reduced when combined with NR. (F) Decreased number of pups per mouse seen in mice fed a high-fat diet compared to control mice and those supplemented with NR.
NAD+ Boosting Undoes Harmful Changes to Oocytes Caused by High-fat Diets
Next, Yang and colleagues looked “under the hood” to see how a high-fat diet could affect oocyte health. On a cellular level, the oocytes of mice fed a high-fat diet appeared structurally altered, had a lower proportion of well-organized chromosomes, and poorly formed spindles – components necessary for cellular division. These issues can negatively affect cell division, critical for oocyte maturation and embryonic development.
On an even more microscopic scale, the researchers observed that reactive oxygen species (ROS) – molecules that cause cellular stress – increased in oocytes of high-fat diet mice. Cellular stress has been shown to affect oocyte maturation negatively and, subsequently, fertility. Finally, the high-fat diet changed oocyte gene activity patterns. Many of these genes were associated with mitochondrial synthesis and function, namely energy production. Furthermore, the levels of adenosine triphosphate (ATP) – an energy molecule primarily made in mitochondria – were decreased in the high-fat diet oocytes, indicative of mitochondria dysfunction.
Ultimately, the high-fat diet-induced NAD+ deficiency in oocytes caused mitochondrial dysfunction, accumulation of ROS, and abnormal spindle assembly. However, increasing NAD+ levels by supplementing high-fat diet mice with NR improved mitochondrial functions in the oocytes. NR supplementation essentially reversed all of these processes – from defects in cell structure and spindles to ROS production and altered gene activity patterns – to undo the damaging effects of a high-fat diet on early embryonic development and the live birth rate.
Intergenerational Effects of NAD+ Boosting
Since mitochondria are inherited from the mother through her oocytes, the investigators considered whether the beneficial effects of NR could be transferred to the mothers’ offspring. The researchers found that this was the case. For example, the glucose levels of offspring from mice fed a high-fat diet were higher than the offspring of control mice following a glucose injection. In contrast, these increased glucose levels were not seen in the offspring of mice supplemented with NR. Additionally, gene activity related to NAD+ levels increased in the offspring of mice fed a high-fat diet supplemented with NR. The researchers suggest that NR supplementation could alleviate obesity-induced metabolic issues in offspring by improving mitochondrial function.
Supporting Evidence for NAD+ Precursors in Fertility
This isn’t the first study on NAD+ precursors and fertility. Numerous studies have shown the positive effects of another NAD+ precursor, nicotinamide mononucleotide (NMN), on oocyte health. Both precursors, NMN and NR, raise the levels of NAD+ within the oocytes, which has been shown to protect against the oxidative stress associated with aging and increased fertility. NMN also protects oocytes against common household cleaners affecting chromosome organization and restores decreased oocyte numbers and fertilization capability associated with aging. Like NR’s ability to preserve oocyte mitochondrial function from a high-fat diet, NMN was also shown to affect oocyte quality by restoring mitochondrial function.
The evidence is mounting that NAD+ precursors may be a viable therapeutic option for preserving and maintaining fertility within an aging population exposed to various sources of cellular stress, including toxins and diet-induced obesity.