Key Points:
- The concentration of naturally-occurring compound spermidine drops by half with age in anticancer immune cells – CD8+ T cells.
- Spermidine supplementation improves CD8+ T cells’ mitochondrial function as shown by increased cell energy molecules – adenosine triphosphate (ATP).
- Supplementing CD8+ T immune cells with spermidine significantly stimulates their tumor-eliminating capabilities when paired with antitumor antibodies.
Supplementing with spermidine, a compound found in all forms of life, has been shown to enhance antitumor activity in animal models. Intriguingly, and possibly partially due to its antitumor properties, spermidine has been shown to extend the mouse lifespan. How exactly spermidine exerts its antitumor effects has remained, for the most part, unclear.
Published in Science, Honjo and colleagues from Kyoto University in Japan show that spermidine supplementation improves the effectiveness of antitumor immune cells when paired with anticancer antibody injections. Spermidine levels decline with age in immune cells, and increasing its levels in anticancer CD8+ T immune cells enhances the cells’ mitochondrial metabolism as shown by increased ATP. Improved anticancer immune cell metabolism provides evidence for the mechanism behind spermidine’s anticancer potential.
Spermidine Supplementation Stimulates Anticancer Immune Cell Metabolism
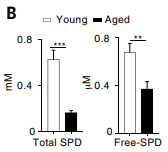
To confirm that spermidine levels drop with age in anticancer immune cells, Honjo and colleagues measured spermidine levels in CD8+ T cells – “killer” white blood cells that eliminate diseased cells like cancer – from young and old mice. The Japanese team chose to analyze CD8+ T cells since these are the primary cells that engulf and destroy cancer cells. They found that the CD8+T cells from older mice had about half the spermidine of their younger counterparts. These findings illustrate that spermidine levels dramatically decline in aged anticancer immune cells.
Honjo and colleagues sought to find how spermidine supplementation may stimulate CD8+ T cell activity, so they examined metabolic function. By analyzing cell energy production pathways, they found that spermidine binds to and stimulates a protein within mitochondria called the mitochondrial trifunctional protein (MTF). MTF helps metabolize fats to generate ATP. Honjo and colleagues found that spermidine supplementation almost doubled mitochondrial ATP levels through spermidine binding to MTF. These findings provide evidence that spermidine improves CD8+ T cell anticancer activity by stimulating mitochondrial function.
Administering Spermidine Inhibits Tumor Growth
The Kyoto-based team injected mice tumor cells, along with spermidine and anticancer antibodies in mice to see if spermidine inhibits tumor growth. They chose the anticancer antibody therapy since the antibodies recognize and latch onto cancer cells and then present them to “killer” CD8+ T cells for elimination. Since nonresponsiveness to this immunotherapy poses a problem for some with cancer, the research team posed the question whether spermidine insufficiency could be a key factor in this treatment’s lack of effectiveness. While the anticancer antibodies significantly reduced tumor volume over the course of about 20 days, adding spermidine impeded tumor growth further. These results support that spermidine supplementation can enhance the effects of antitumor antibody treatments by enhancing anticancer immune cell mitochondrial metabolism, at least in mice.
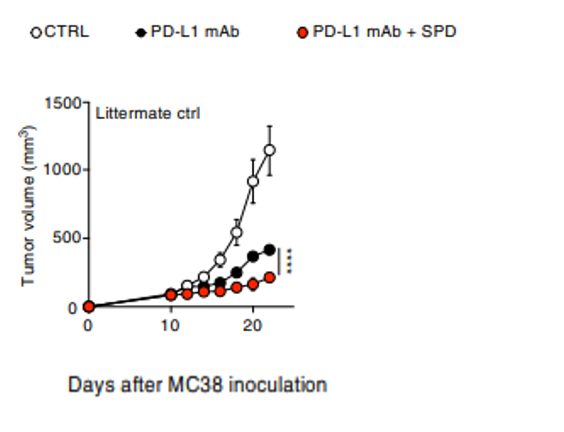
The study provides evidence that spermidine may enhance the effects of anticancer antibody therapies by improving cell energy generation within anticancer CD8+ T immune cells. Other research has pointed to the nicotinamide adenine dinucleotide (NAD+) precursor nicotinamide mononucleotide (NMN) enhancing other anticancer treatments. Future studies could look at whether NMN and spermidine synergistically enhance CD8+ T cell function since NMN is known to also increase ATP levels. Whether these findings apply to humans is also a big question that needs answering.